SI
From Wikipedia, the free encyclopedia.
- For other uses, see SI (disambiguation).
The International System of Units (abbreviated SI from the French language name Système International d'Unités) is the modern form of the metric system. It is the world's most widely used system of units, both in everyday commerce and in science.
The older metric system included several groupings of units. The SI was developed in 1960 from one of these, the metre-kilogram-second (MKS) system, rather than the centimetre-gram-second (CGS) system, which, in turn, had many variants.
The SI introduced several newly named units. The SI is not static; it is a living set of standards where units are created and definitions are modified with international agreement as measurement technology progresses.
With few exceptions (such as draught beer sales in the United Kingdom), the system is legally being used in every country in the world, and many countries do not maintain official definitions of other units. In the United States, industrial use of SI is increasing, but popular use is still limited. In the United Kingdom, conversion to metric units is official policy but not yet complete. Those countries that still recognize non-SI units (e.g. the US and UK) have redefined most of their traditional, non-SI units in terms of SI units.
Contents |
History
The metric system was officially adopted in France after the French Revolution. During the history of the metric system a number of variations have evolved and their use spread around the world replacing many traditional measurement systems.
By the end of World War II a number of different systems of measurement were still in use throughout the world. Some of these systems were metric system variations whilst others were based on the Imperial and American systems. It was recognised that additional steps were needed to promote a worldwide measurement system. As a result the 9th General Conference on Weights and Measures (CGPM), in 1948, asked the International Committee for Weights and Measures (CIPM) to conduct an international study of the measurement needs of the scientific, technical, and educational communities.
Based on the findings of this study, the 10th CGPM in 1954 decided that an international system should be derived from six base units to provide for the measurement of temperature and optical radiation in addition to mechanical and electromagnetic quantities. The six base units recommended were the metre, kilogram, second, ampere, Kelvin degree (later renamed the kelvin), and the candela. In 1960, the 11th CGPM named the system the International System of Units, abbreviated SI from the French name: Le Système International d'Unités. The seventh base unit, the mole, was added in 1970 by the 14th CGPM.
The International System is now either obligatory or permissible throughout the world. It is administered by the standards organisation: the Bureau International des Poids et Mesures (International Bureau of Weights and Measures).
Units
- Main articles: SI base unit, SI derived unit, SI prefix
The international system of units consists of a set of units together with a set of prefixes. The units of SI can be divided into two subsets. There are the seven base units. Each of these base units are dimensionally independent. From these seven base units several other units are derived. In addition to the SI units there are also a set of non-SI units accepted for use with SI.
| SI base units | ||
|---|---|---|
| Name | Symbol | Quantity |
| kilogram | kg | Mass |
| second | s | Time |
| metre | m | Length |
| ampere | A | Electrical current |
| kelvin | K | Temperature |
| mole | mol | Amount of substance |
| candela | cd | Luminous intensity |
A prefix may be added to units to produce a multiple of the original unit. All multiples are integer powers of ten. For example, kilo- denotes a multiple of a thousand and milli- denotes a multiple of a thousandth hence there are one thousand millimetres to the metre and one thousand metres to the kilometre. The prefixes are never combined: a millionth of a kilogram is a milligram not a microkilogram.
SI writing style
- Symbols are written in lower case, except for symbols derived from the name of a person. For example, the unit of pressure is named after Blaise Pascal, so its symbol is written "Pa" whereas the unit itself is written "pascal". The one exception is the litre, whose original abbreviation "l" is dangerously similar to "1". The NIST recommends that "L" be used instead, a usage which is common in the U.S., Canada and Australia, and has been accepted as an alternative by the CGPM. The cursive "ℓ" is occasionally seen, especially in Japan, but this is not currently recommended by any standards body. For more information, see Litre.
- Symbols are written without grammatical markers when used with singular numerals: i.e. "25 kg", not "25 kgs". Pluralization would be language dependent; "s" plurals (as in French and English) are particularly undesirable since "s" is the symbol of the second. Other cases may be marked in a language-dependent manner, e.g. Finnish 25 kg:lla = 25 kilogrammalla "with 25 kg".
- Symbols do not have an appended period (.).
- It is preferable to write symbols in upright Roman type (m for metres, L for litres), so as to differentiate from the italic type used for mathematical variables (m for mass, l for length).
- A space should separate the number and the symbol, e.g. "2.21 kg", "7.3×102 m2", "22 °C" [1]. Exceptions are the symbols for plane angular degrees, minutes and seconds (°, ′ and ″), which are placed immediately after the number with no intervening space.
- Spaces should be used to group decimal digits in threes, e.g. 1 000 000 or 342 142 (in contrast to the commas or dots used in other systems, e.g. 1,000,000 or 1.000.000).
- The 10th resolution of CGPM in 2003 declared that "the symbol for the decimal marker shall be either the point on the line or the comma on the line". In practice, the full stop is used in English, and the comma in most other European languages.
- Symbols for derived units formed from multiple units by multiplication are joined with a space or centre dot (·), e.g. N m or N·m.
- Symbols formed by division of two units are joined with a solidus (/), or given as a negative exponent. For example, the "metre per second" can be written "m/s", "m s-1", "m·s-1" or
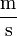 . A solidus should not be used if the result is ambiguous, i.e. "kg·m-1·s-2" is preferable to "kg/m/s2".
. A solidus should not be used if the result is ambiguous, i.e. "kg·m-1·s-2" is preferable to "kg/m/s2".
Spelling variations
- Several nations, notably the United States, typically use the spellings 'meter' and 'liter' instead of 'metre' and 'litre' in keeping with standard American English spelling. In addition, the official US spelling for the SI prefix 'deca' is 'deka'.
- The unit 'gram' is also sometimes spelled 'gramme' in English-speaking countries other than the United States, though that is an older spelling and use is declining.
Cultural issues
The swift worldwide adoption of the metric system as a tool of economy and everyday commerce was based mainly on the lack of customary systems in many countries to adequately describe some concepts, or as a result of an attempt to standardize the many regional variations in the customary system. International factors also affected the adoption of the metric system, as many countries increased their trade. Scientifically, it provides ease when dealing with very large and small quantities because it lines up so well with our decimal numeral system.
Cultural differences can be represented in the local everyday uses of metric units. For example, bread is sold in one-half, one or two kilogram sizes in many countries, but you buy them by multiples of one hundred grams in the former USSR. In some countries, the informal cup measurement has become 250 mL, and prices for items are sometimes given per 100 g rather than per kilogram. A profound cultural difference between physicists and engineers, especially radio engineers, existed prior to the adoption of the metre-kilogram-second (MKS) system and hence its descendent, SI. Engineers work with volts, amperes, ohms, farads, and coulombs, which are of great practical utility, while the centimetre-gram-second (CGS) units, which are fine for theoretical physics can be inconvenient for electrical engineering usage and are largely unfamiliar to householders using appliances rated in volts and watts. People with diabetes test their plasma glucose level regularly. In the U.S., measurement are recorded in milligrams per deciliter (mg/dL), in Europe, the standard is millimole/liter (mmol/L).
The fine-tuning that has happened to the metric base units over the past 200 years, as experts have tried periodically to refine the metric system to fit the best scientific research do not affect the everyday use of metric units. Since most non-SI units, such as the U.S. customary units, are nowadays defined in terms of SI units, any change in the definition of the SI units results in a change of the definition of the older units as well.
See also
- Units of measurement
- Weights and measures
- Mesures usuelles
- Metrified English unit
- History of measurement
- Other systems of measurement:
- CODATA
- Metrication
- Metric system in the United States
- Metrology
- UTC (Coordinated Universal Time)
- Binary prefixes - used to quantify large amounts of computer data
- Orders of magnitude
- ISO 31
External links
Official
- BIPM (SI maintenance agency) (home page)
- BIPM brochure (SI reference)
- ISO 1000:1992 SI units and recommendations for the use of their multiples and of certain other units, with its price tag of 99 Swiss francs for a 22 page, coverless pamphlet showing why the public is sometimes a little slow to pick up on their recommendations.
Information
- US NIST reference on SI
- SI - Its history and use in science and industry
- A Dictionary of Units of Measurement
- Cyrillic transcription of SI symbols
- Judson, Lewis B., Weights and Measures Standards of the United States: A brief history, NBS Special Publication 447, orig. iss. October 1963, updated March 1976 (46 page PDF file)
- Metric system and conversion tables (courtesy French property advice)
- metre-info - an encyclopaedia of all metric units
Pro-metric pressure groups
Pro-customary measures pressure groups
Further reading
- I. Mills, Tomislav Cvitas, Klaus Homann, Nikola Kallay, IUPAC: Quantities, Units and Symbols in Physical Chemistry, 2nd ed., Blackwell Science Inc 1993, ISBN 0632035838.

