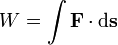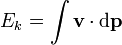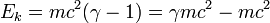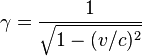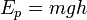Energy
From Wikipedia, the free encyclopedia.
In physics, energy is a fundamental quantity that every physical system possesses. Energy of physical system in a certain given state is defined as the amount of work (W) needed to change the state of the system from some initial position, known as the reference state or reference level, to a specific or final position.
For example, it takes W = (1/2)mv² amount of work to accelerate a bullet from zero speed to speed v — thus the quantity (1/2)mv² is called a kinetic energy of a bullet. Or it takes W = mgh amount of work to elevate mass m at height h in gravitational field g, thus the amount mgh is called gravitational potential energy.
Other examples are the electrical energy stored in a battery, the chemical energy stored in a piece of food, the thermal energy of a water heater, or the potential energy of elevated water behind a dam.
Energy can be readily transformed from one form into another; for instance, using a battery to power an electrical heater converts chemical energy into electrical energy, which is then converted into thermal energy. Or letting elevated water move down transforms stored potential energy into kinetic energy of moving water and turbine, which in turn transforms into electric energy by generator. The law of conservation of energy states that in a closed system the total amount of energy, corresponding to the sum of a system's constituent energy components, remains constant. This law follows from translational symmetry of time (=independence of any physical process on the moment it started). Some works (thus some forms of energy) are not easily measured by the unaided observer.
Energy can also be used in a spiritual or non-scientific way that cannot be quantified. Usually this has something to do with mystical and/or healing type references such as acupuncture and witch doctors.
Contents |
Forms of Energy
- Kinetic energy: the energy of moving objects
- Thermal energy: the energy associated with heat
- Sound energy: the energy of compression waves
- Electrical energy: the energy of moving charged particles
- Potential Energy: the energy that an object has due to position; also known as stored energy
- Chemical energy: the stored energy of chemical substances
- Nuclear energy: the stored energy of the atomic nucleus
- Radiant energy: the energy of electromagnetic waves, including light
Units
SI
Hi The SI unit for both energy and work is the Joule (J), named in honour of James Prescott Joule and his experiments on the mechanical equivalent of heat. In slightly more fundamental terms, 1 joule is equal to 1 Newton-meter and, in terms of SI base units:
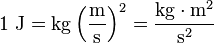
An energy unit that is used in particle physics is the electronvolt (eV). One eV is equivalent to 1.60217653×10−19 J.
In spectroscopy the unit cm − 1 = 0.0001239eV is used to represent energy since energy is inversely proportional to wavelength from the equation E = hν = hc / λ.
(Note that torque, which is typically expressed in newton-metres, has the same dimension and this is not a simple coincidence: a torque of 1 newton-metre applied on 1 radian requires exactly 1 newton-metre=joule of energy.)
Other units of energy
In cgs units, one erg is 1 g cm2 s−2, equal to 1.0×10−7 J. Another obsolete metric unit is the litre-atmosphere (101.325 J).
The imperial/US units for both energy and work include the foot-pound force (1.3558 J), the British thermal unit (Btu) which has various values in the region of 1055 J, and the horsepower-hour (2.6845 MJ).
The energy unit used for everyday electricity, particularly for utility bills, is the kilowatt-hour (kW h), and one kW h is equivalent to 3.6×106 J (3600 kJ or 3.6 MJ; the metric units usually are self-consistent, and this particular one may seem arbitrary; it's not, the metric measurement for time is the second, and there are 3,600 seconds in an hour -- in other words, 1 kW second = 1 kJ, but the kW h is a more convenient unit for everyday use).
The calorie is mainly used in nutrition and equals the amount of heat necessary to raise the temperature of one kilogram of water by 1 degree Celsius, at a pressure of 1 atm. This amount of heat depends somewhat on the initial temperature of the water, which results in various different units sharing the name of "calorie" but having slightly different energy values. It is equal to 4.1868 kJ.
The calories used for food energy in nutrition are the large calories based on the kilogram rather than the gram, often identified as food calories. These are sometimes called kilocalories with that calorie being the small calorie based on the gram, and as a result the prefixes are generally avoided for the large calories (i.e., 1 kcal is 4.184 kJ, never 4.184 MJ, even if "calories" are also used for the other, larger unit in the same document or the same nutrition label). Food calories are sometimes noted as Calories (1000 calories) or simply abbreviated Cal with the capital C, but that convention is more often found in chemistry or physics textbooks—which do not use these large calories—than it is in real-world applications by those who do use these calories. (This convention is also, of course, useless when the word calorie appears in a location where it would ordinarily be capitalized, as at the beginning of a sentence or in the first column of a nutrition label as a substitute for the quantity being measured, which is energy, when all the other quantities such as "Iron" and "Sugars" are also capitalized.)
Transfer of energy
Work
Main article: mechanical work.
Work is a defined as a path integral of force F over distance s:
The equation above says that the work (W) is equal to the integral of the dot product of the force ( ) on a body and the infinitesimal of the body's position (
) on a body and the infinitesimal of the body's position ( ).
).
Heat
Main article: Heat.
Heat is an amount of energy which is usually linked with a change in temperature or in a change in phase of matter. In chemistry, heat is the amount of energy which is absorbed or released by a given chemical reaction. The relationship between heat and energy is similar to that between work and energy. Heat flows from areas of high temperature to areas of low temperature. All objects (matter) have a certain amount of internal energy that is related to the random motion of their atoms or molecules. This internal energy is directly proportional to the temperature of the object. When two bodies of different temperature come in to thermal contact, they will exchange internal energy until the temperature is equalised. The amount of energy transferred is the amount of heat exchanged. It is a common misconception to confuse heat with internal energy, but there is a difference: the change of the internal energy is the heat that flows from the surroundings into the system plus the work performed by the surroundings on the system. Heat Energy is transferred in three different ways: conduction, convection and/or radiation.
Conservation of energy
The first law of thermodynamics says that the total inflow of energy into a system must equal the total outflow of energy from the system, plus the change in the energy contained within the system. This law is used in all branches of physics, but frequently violated by quantum mechanics (see off shell). Noether's theorem relates the conservation of energy to the time invariance of physical laws.
An example of the conversion and conservation of energy is a pendulum. At its highest points the kinetic energy is zero and the potential gravitational energy is at its maximum. At its lowest point the kinetic energy is at its maximum and is equal to the decrease of potential energy. If one unrealistically assumes that there is no friction, the energy will be conserved and the pendulum will continue swinging forever. (In practice, available energy is never perfectly conserved when a system changes state; otherwise, the creation of perpetual motion machines would be possible.)
Another example is a chemical explosion in which potential chemical energy is converted to kinetic energy and heat in a very short time.
Types of energy
Kinetic energy
Main article: Kinetic energy.
Kinetic energy is the portion of energy related to the motion of a body.
The equation above says that the kinetic energy (Ek) is equal to the integral of the dot product of the velocity (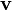 ) of a body and the infinitesimal of the body's momentum (
) of a body and the infinitesimal of the body's momentum (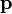 ).
).
For non-relativistic velocities, that is velocities much smaller than the speed of light, we can use the Newtonian approximation
where
Ek is kinetic energy
m is mass of the body
v is velocity of the body
At near-light velocities, we use the correct relativistic formula:
where
v is the velocity of the body
m is its rest mass
c is the speed of light in a vacuum, which is approximately 300,000 kilometers per second
 is the total energy of the body
is the total energy of the body
 is again the rest mass energy.
is again the rest mass energy.
See also, E=mc².
In the form of a Taylor series, the relativistic formula for can be written as:
Hence, the second and higher terms in the series correspond with the "inaccuracy" of the Newtonian approximation for kinetic energy in relation to the relativistic formula.
Potential energy
Main article: Potential energy.
In contrast to kinetic energy, which is the energy of a system due to its motion, or the internal motion of its particles, the potential energy of a system is the energy associated with the spatial configuration of its components and their interaction with each other. Any number of particles which exert forces on each other automatically constitute a system with potential energy. Such forces, for example, may arise from electrostatic interaction (see Coulomb's law), or gravity.
In an isolated system consisting of two stationary objects that exert a force f(x) on each other and lay on the x-axis, their potential energy is most generally defined as
where the force between the objects varies only with distance x and is integrated along the line connecting the two objects.
To further illustrate the relationship between force and potential energy, consider the same system of two objects situated along the x-axis. If the potential energy due to one of the objects at any point x is U(x), then the force on the that object x is
This mathematical relationship demonstrates the direct connection between force and potential energy: the force between two objects is in the direction of decreasing potential energy, and the magnitude of the force is proportional to the extent to which potential energy decreases. A large force is associated with a large decrease in potential energy, while a small force is associated with a small decrease in potential energy. Notice how, in this case, the force on an object depends entirely on its potential energy.
These two relationships – the definition of potential energy based on force, and the dependence of force on potential energy – show how the concepts of force and potential energy are intimately linked: if two objects do not exert forces on each other, there is no potential energy between them. If two objects do exert forces on each other, then potential energy naturally arises in the system as part of the system's total energy. Since potential energy arises from forces, any change in the system's spatial configuration will either increase or decrease the system's potential energy as the objects are repositioned.
When a system moves to a lower potential energy state, energy is either released in some form or converted into another form of energy, such as kinetic energy. The potential energy can be "stored" as gravitational energy, elastic energy, chemical energy, rest mass energy or electrical energy, but arises in all cases from the spatial positioning and interaction of objects within a system. Unlike kinetic energy, which exists in any moving body, potential energy exists in any body which is interacting with another object.
For example a mass released above the Earth initially has potential energy resulting from the gravitational attraction of the Earth, which is transferred to kinetic energy as the gravitational force acts on the object and its potential energy is decreased as it falls.
Equation:
where m is the mass, h is the height and g is the value of acceleration due to gravity at the Earth's surface (see gee).
Internal energy
Main article: Internal energy.
Internal energy is the kinetic energy associated with the motion of molecules, and the potential energy associated with the rotational, vibrational and electric energy of atoms within molecules. Internal energy, like energy, is a quantifiable state function of a system.
History
In the past, energy was discussed in terms of easily observable effects it has on the properties of objects or changes in state of various systems. Basically, if something changed, some sort of energy was involved in that change. As it was realized that energy could be stored in objects, the concept of energy came to embrace the idea of the potential for change as well as change itself. Such effects (both potential and realized) come in many different forms; examples are the electrical energy stored in a battery, the chemical energy stored in a piece of food, the thermal energy of a water heater, or the kinetic energy of a moving train. To simply say energy is "change or the potential for change", however, misses many important examples of energy as it exists in the physical world.
The concept of energy and work are relatively new additions to the physicist’s toolbox. Neither Galileo nor Newton made any contributions to the theoretical model of energy, and it was not until the middle of the 19th century that these concepts were introduced.
The development of steam engines required engineers to develop concepts and formulas that would allow them to describe the mechanical and thermal efficiencies of their systems. Engineers such as Sadi Carnot and James Prescott Joule, mathematicians such as Émile Claperyon and Hermann von Helmholtz , and amateurs such as Julius Robert von Mayer all contibuted to the notions that the ability to perform certain tasks, called work, was somehow related to the amount of energy in the system. The nature of energy was elusive, however, and it was argued for some years whether energy was a substance (the caloric) or merely a physical quantity, such as momentum.
William Thomson (Lord Kelvin) amalgamated all of these laws into his laws of thermodynamics, which aided in the rapid development of energetic descriptions of chemical processes by Rudolf Clausius, Josiah Willard Gibbs, Walther Nernst. In addition, this allowed Ludwig Boltzmann to describe entropy in mathematical terms, and to discuss, along with Jožef Stefan, the laws of radiant energy.
For further information, see the Timeline of thermodynamics.
Energy use
Main articles: energy development, energy policy
The way in which humans use energy is one of the defining characteristics of an economy. The progression from animal power to steam power, then the internal combustion engine and electricity, are key elements in the development of modern civilization. Future energy development, for example of renewable energy, may be key to avoiding the effects of global warming.
See also
Energy in natural sciences
- energy conversion
- enthalpy
- exergy
- power (physics)
- specific orbital energy
- solar radiation
- thermodynamics
- thermodynamic entropy
Energy use by humans
Major topics
- List of energy topics
- Embodied energy or emergy
- Energy crisis
- Energy development
- Energy policy
- Renewable energy
Other articles
- Energy balance
- Energy demand management and DSM
- Energy storage
- Energy transmission
- EU Energy Label
- EU Intelligent Energy, energy efficiency and renewables.
Further reading
- Feynman, Richard. Six Easy Pieces: Essentials of Physics Explained by Its Most Brilliant Teacher. Helix Book. See the chapter "conservation of energy" for Feynman's explanation of what energy is and how to think about it.
- Einstein, Albert (1952). Relativity: The Special and the General Theory (Fifteenth Edition). ISBN 0-517-88441-0
External links
- Online Energy and Work Converter - convert between various units of energy and work, such as joule, erg, gigawatt-hour, newton meter, calorie, Btu, and so on
- Interactive Energy and Work Conversion Table - convert selected unit to all other units of energy and work